What Are Damages Or Changes To A Dna Sequence Called
DNA damage is distinctly different from mutation, although both are types of error in Dna. DNA damage is an abnormal chemical structure in Dna, while a mutation is a alter in the sequence of base pairs. Deoxyribonucleic acid damages crusade changes in the construction of the genetic material and prevents the replication mechanism from functioning and performing properly.[i]
Dna damage and mutation have unlike biological consequences. While almost DNA damages can undergo DNA repair, such repair is not 100% efficient. United nations-repaired DNA amercement accumulate in non-replicating cells, such every bit cells in the brains or muscles of adult mammals, and can cause aging.[2] [three] [4] (Likewise see DNA damage theory of aging.) In replicating cells, such as cells lining the colon, errors occur upon replication past damages in the template strand of DNA or during repair of DNA amercement. These errors can give rise to mutations or epigenetic alterations.[5] Both of these types of alteration can exist replicated and passed on to subsequent prison cell generations. These alterations can change gene function or regulation of gene expression and possibly contribute to progression to cancer.
Throughout the jail cell bike at that place are various checkpoints to ensure the prison cell is in good condition to progress to mitosis. The 3 main checkpoints are at G1/s, G2/grand, and at the spindle associates checkpoint regulating progression through anaphase. G1 and G2 checkpoints involve scanning for damaged DNA.[6] During Southward stage the cell is more vulnerable to Dna damage than any other part of the cell cycle. G2 checkpoint checks for damaged DNA and Dna replication completeness. Deoxyribonucleic acid harm is an alteration in the chemical construction of DNA, such as a suspension in a strand of DNA, a base of operations missing from the backbone of Deoxyribonucleic acid, or a chemically changed base such every bit eight-OHdG. DNA damage can occur naturally or via ecology factors. The Dna damage response (DDR) is a complex signal transduction pathway which recognizes when DNA is damaged and initiates the cellular response to the damage.[7]
Types [edit]
Damage to Deoxyribonucleic acid that occurs naturally can event from metabolic or hydrolytic processes. Metabolism releases compounds that harm Deoxyribonucleic acid including reactive oxygen species, reactive nitrogen species, reactive carbonyl species, lipid peroxidation products and alkylating agents, amongst others, while hydrolysis cleaves chemic bonds in DNA.[eight] Naturally occurring oxidative Deoxyribonucleic acid amercement arise at least 10,000 times per jail cell per 24-hour interval in humans and as much as 100,000 per cell per twenty-four hour period in rats[nine] as documented below.
Oxidative Deoxyribonucleic acid damage tin produce more than than 20 types of altered bases[10] [11] besides equally unmarried strand breaks.[12]
Other types of endogeneous DNA damages, given beneath with their frequencies of occurrence, include depurinations, depyrimidinations, double-strand breaks, O6-methylguanines and cytosine deamination.
Dna tin be damaged via ecology factors as well. Ecology agents such as UV light, ionizing radiations, and genotoxic chemicals. Replication forks can be stalled due to damaged Deoxyribonucleic acid and double strand breaks are also a form of Dna damage.[13]
Frequencies [edit]
The list below shows some frequencies with which new naturally occurring DNA amercement ascend per solar day, due to endogenous cellular processes.
- Oxidative damages
- Humans, per cell per twenty-four hours
- 10,000[9]
11,500[14]
ii,800[15] specific amercement 8-oxoGua, 8-oxodG plus 5-HMUra
two,800[xvi] specific damages eight-oxoGua, 8-oxodG plus v-HMUra
- 10,000[9]
- Rats, per cell per day
- 74,000[14]
86,000[17]
100,000[nine]
- 74,000[14]
- Mice, per prison cell per day
- 34,000[15] specific damages 8-oxoGua, 8-oxodG plus v-HMUra
47,000[18] specific damages oxo8dG in mouse liver
28,000[16] specific amercement eight-oxoGua, 8-oxodG, five-HMUra
- 34,000[15] specific damages 8-oxoGua, 8-oxodG plus v-HMUra
- Humans, per cell per twenty-four hours
- Depurinations
- Mammalian cells, per cell per mean solar day
- two,000 to 10,000[19] [20]
ix,000[21]
12,000[22]
13,920[23]
- two,000 to 10,000[19] [20]
- Mammalian cells, per cell per mean solar day
- Depyrimidinations
- Mammalian cells, per cell per twenty-four hour period
- 600[22]
696[23]
- 600[22]
- Mammalian cells, per cell per twenty-four hour period
- Single-strand breaks
- Mammalian cells, per jail cell per solar day
- 55,200[23]
- Mammalian cells, per jail cell per solar day
- Double-strand breaks
- Human cells, per cell cycle
- 10[24]
50[25]
- 10[24]
- Human cells, per cell cycle
- O6-methylguanines
- Mammalian cells, per cell per 24-hour interval
- 3,120[23]
- Mammalian cells, per cell per 24-hour interval
- Cytosine deamination
- Mammalian cells, per cell per day
- 192[23]
- Mammalian cells, per cell per day
Another of import endogenous DNA damage is M1dG, short for (3-(two'-deoxy-beta-D-erythro-pentofuranosyl)-pyrimido[1,2-a]-purin-10(3H)-i). The excretion in urine (likely reflecting charge per unit of occurrence) of M1dG may be as much as 1,000-fold lower than that of 8-oxodG.[26] However, a more important measure may be the steady-country level in Dna, reflecting both rate of occurrence and rate of Dna repair. The steady-state level of M1dG is college than that of 8-oxodG.[27] This points out that some DNA damages produced at a low rate may be hard to repair and remain in DNA at a high steady-land level. Both M1dG[28] and 8-oxodG[29] are mutagenic.
Steady-state levels [edit]
Steady-state levels of DNA amercement represent the balance between formation and repair. More 100 types of oxidative Deoxyribonucleic acid damage have been characterized, and viii-oxodG constitutes almost five% of the steady state oxidative amercement in Deoxyribonucleic acid.[18] Helbock et al.[14] estimated that at that place were 24,000 steady state oxidative Dna adducts per cell in immature rats and 66,000 adducts per cell in former rats. This reflects the aggregating of DNA damage with age. Dna damage accumulation with age is farther described in Dna harm theory of aging.
Swenberg et al.[thirty] measured average amounts of selected steady state endogenous DNA damages in mammalian cells. The vii most common amercement they evaluated are shown in Table 1.
| Endogenous lesions | Number per cell |
|---|---|
| Abasic sites | 30,000 |
| N7-(2-hydroxethyl)guanine (7HEG) | 3,000 |
| 8-hydroxyguanine | two,400 |
| 7-(ii-oxoethyl)guanine | one,500 |
| Formaldehyde adducts | 960 |
| Acrolein-deoxyguanine | 120 |
| Malondialdehyde-deoxyguanine | sixty |
Evaluating steady-state damages in specific tissues of the rat, Nakamura and Swenberg[31] indicated that the number of abasic sites varied from near 50,000 per cell in liver, kidney and lung to about 200,000 per cell in the brain.
Biomolecular pathways [edit]
Proteins promoting endogenous DNA harm were identified in a 2019 paper as the DNA "harm-up" proteins (DDPs).[32] The DDP mechanisms fall into iii clusters:
- reactive oxygen increase by transmembrane transporters,
- chromosome loss by replisome binding,
- replication stalling by transcription factors.[32]
The DDP human homologs are over-represented in known cancer drivers, and their RNAs in tumors predict heavy mutagenesis and a poor prognosis.[32]
Repair of damaged Deoxyribonucleic acid [edit]
In the presence of Deoxyribonucleic acid damage, the cell tin either repair the damage or induce cell decease if the harm is beyond repair.
Types [edit]
The seven chief types of DNA repair and one pathway of impairment tolerance, the lesions they address, and the accuracy of the repair (or tolerance) are shown in this table. For a brief description of the steps in repair see DNA repair mechanisms or see each individual pathway.
| Repair pathway | Lesions | Accuracy | Ref. |
|---|---|---|---|
| Base excision repair | corrects DNA damage from oxidation, deamination and alkylation, also single-strand breaks | authentic | [33] [34] |
| Nucleotide excision repair | oxidative endogenous lesions such every bit cyclopurine, sunlight-induced thymine dimers (cyclobutane dimers and pyrimidine (six-4) pyrimidone photoproducts) | accurate | [35] [36] [37] |
| Homology-directed repair | double-strand breaks in the mid-S phase or mid-G2 stage of the prison cell cycle | authentic | [38] |
| Non-homologous terminate joining | double-strand breaks if cells are in the G0 stage. the G1 phase or the G2 phase of the cell cycle | somewhat inaccurate | [38] |
| Microhomology-mediated end joining or alt-Finish joining | double-strand breaks in the S stage of the cell cycle | ever inaccurate | [38] |
| DNA mismatch repair | base of operations exchange mismatches and insertion-deletion mismatches generated during DNA replication | authentic | [39] |
| Direct reversal (MGMT and AlkB) | 6-O-methylguanine is reversed to guanine by MGMT, some other methylated bases are demethylated by AlkB | accurate | [forty] |
| Translesion synthesis | DNA harm tolerance process that allows the DNA replication machinery to replicate past Deoxyribonucleic acid lesions | may be inaccurate | [41] |
Crumbling and cancer [edit]

Dna impairment in non-replicating cells, if not repaired and accumulated can lead to aging. DNA damage in replicating cells, if not repaired tin pb to either apoptosis or to cancer.
The schematic diagram indicates the roles of insufficient Deoxyribonucleic acid repair in crumbling and cancer, and the role of apoptosis in cancer prevention. An excess of naturally occurring Dna impairment, due to inherited deficiencies in particular DNA repair enzymes, tin can cause premature aging or increased chance for cancer (come across DNA repair-deficiency disorder). On the other hand, the ability to trigger apoptosis in the presence of backlog un-repaired Deoxyribonucleic acid damage is critical for prevention of cancer.[42]
Apoptosis and cancer prevention [edit]
Deoxyribonucleic acid repair proteins are often activated or induced when Dna has sustained damage. Notwithstanding, excessive Dna damage tin can initiate apoptosis (i.eastward., programmed jail cell death) if the level of DNA damage exceeds the repair capacity. Apoptosis can forestall cells with excess DNA damage from undergoing mutagenesis and progression to cancer.[43]
Inflammation is often acquired by infection, such as with hepatitis B virus (HBV), hepatitis C virus (HCV) or Helicobacter pylori. Chronic inflammation is also a primal characteristic of obesity.[44] [45] [46] [47] Such inflammation causes oxidative DNA damage. This is due to the induction of reactive oxygen species (ROS) by various intracellular inflammatory mediators.[48] [49] [50] HBV and HCV infections, in detail, crusade 10,000-fold and 100,000-fold increases in intracellular ROS production, respectively.[51] Inflammation-induced ROS that cause Dna damage tin can trigger apoptosis,[52] [53] only may also crusade cancer if repair and apoptotic processes are comparatively protective.[45]
Bile acids, stored in the gall bladder, are released into the modest intestine in response to fatty in the diet. Higher levels of fatty cause greater release.[54] Bile acids cause Deoxyribonucleic acid damage, including oxidative Dna damage, double-strand Dna breaks, aneuploidy and chromosome breakage.[55] High-normal levels of the bile acid deoxycholic acid crusade apoptosis in human colon cells,[56] but may besides lead to colon cancer if repair and apoptotic defenses are insufficient.[57]
Apoptosis serves as a safeguard mechanism against tumorigenesis.[58] It prevents the increased mutagenesis that excess Deoxyribonucleic acid impairment could cause, upon replication.[59]
At least 17 Deoxyribonucleic acid repair proteins, distributed among 5 Dna repair pathways, accept a "dual function" in response to Deoxyribonucleic acid damage. With moderate levels of DNA damage, these proteins initiate or contribute to Deoxyribonucleic acid repair. However, when excessive levels of Deoxyribonucleic acid damage are present, they trigger apoptosis.[43]
DNA damage response [edit]
The packaging of eukaryotic Dna into chromatin is a bulwark to all DNA-based processes that require enzyme action. For most DNA repair processes, the chromatin must be remodeled. In eukaryotes, ATP-dependent chromatin remodeling complexes and histone-modifying enzymes are two factors that act to achieve this remodeling procedure later DNA harm occurs.[60] Further DNA repair steps, involving multiple enzymes, usually follow. Some of the first responses to DNA harm, with their timing, are described beneath. More complete descriptions of the Dna repair pathways are presented in articles describing each pathway. At least 169 enzymes are involved in DNA repair pathways.[61]
Base excision repair [edit]
Oxidized bases in Dna are produced in cells treated with Hoechst dye followed by micro-irradiation with 405 nm light.[62] Such oxidized bases can be repaired by base excision repair.
When the 405 nm light is focused along a narrow line within the nucleus of a prison cell, near two.5 seconds after irradiation, the chromatin remodeling enzyme Alc1 achieves half-maximum recruitment onto the irradiated micro-line.[63] The line of chromatin that was irradiated and then relaxes, expanding side-to-side over the side by side 60 seconds.[63]
Within six seconds of the irradiation with 405 nm calorie-free, there is half-maximum recruitment of OGG1 to the irradiated line.[62] OGG1 is an enzyme that removes the oxidative Dna damage viii-oxo-dG from DNA. Removal of 8-oxo-dG, during base excision repair, occurs with a half-life of 11 minutes.[18]
Nucleotide excision repair [edit]
Ultraviolet (UV) light induces the formation of Deoxyribonucleic acid amercement including pyrimidine dimers (such equally thymine dimers) and 6,four photoproducts. These types of "bulky" damages are repaired past nucleotide excision repair.
Afterwards irradiation with UV light, DDB2, in a complex with DDB1, the ubiquitin ligase protein CUL4A and the Band finger protein ROC1, assembly with sites of damage within chromatin. Half-maximum association occurs in 40 seconds.[64] PARP1 also associates inside this period.[65] The PARP1 protein attaches to both DDB1 and DDB2 and then PARylates (creates a poly-ADP ribose chain) on DDB2 that attracts the Dna remodeling protein ALC1.[65] ALC1 relaxes chromatin at sites of UV damage to Dna. In addition, the ubiquitin E3 ligase complex DDB1-CUL4A carries out ubiquitination of the core histones H2A, H3, and H4, equally well as the repair protein XPC, which has been attracted to the site of the Deoxyribonucleic acid impairment.[66] XPC, upon ubiquitination, is activated and initiates the nucleotide excision repair pathway. Somewhat subsequently, at 30 minutes subsequently UV impairment, the INO80 chromatin remodeling circuitous is recruited to the site of the Dna damage, and this coincides with the bounden of farther nucleotide excision repair proteins, including ERCC1.[67]
Homologous recombinational repair [edit]
Double-strand breaks (DSBs) at specific sites can be induced by transfecting cells with a plasmid encoding I-SceI endonuclease (a homing endonuclease). Multiple DSBs can exist induced past irradiating sensitized cells (labeled with five'-bromo-two'-deoxyuridine and with Hoechst dye) with 780 nm calorie-free. These DSBs tin be repaired past the accurate homologous recombinational repair or by the less accurate non-homologous end joining repair pathway. Here nosotros depict the early on steps in homologous recombinational repair (HRR).
After treating cells to innovate DSBs, the stress-activated protein kinase, c-Jun North-terminal kinase (JNK), phosphorylates SIRT6 on serine 10.[68] This post-translational modification facilitates the mobilization of SIRT6 to DNA harm sites with half-maximum recruitment in well under a 2nd.[68] SIRT6 at the site is required for efficient recruitment of poly (ADP-ribose) polymerase 1 (PARP1) to a DNA break site and for efficient repair of DSBs.[68] PARP1 poly peptide starts to appear at DSBs in less than a second, with half maximum aggregating within ane.6 seconds after the damage occurs.[69] This then allows half maximum recruitment of the Dna repair enzymes MRE11 within 13 seconds and NBS1 inside 28 seconds.[69] MRE11 and NBS1 carry out early steps of the HRR pathway.
γH2AX, the phosphorylated course of H2AX is also involved in early steps of DSB repair. The histone variant H2AX constitutes virtually ten% of the H2A histones in homo chromatin.[lxx] γH2AX (H2AX phosphorylated on serine 139) can be detected as soon as 20 seconds afterwards irradiation of cells (with DNA double-strand break formation), and one-half maximum accumulation of γH2AX occurs in one minute.[lxx] The extent of chromatin with phosphorylated γH2AX is about ii million base pairs at the site of a DNA double-strand interruption.[seventy] γH2AX does not, itself, cause chromatin decondensation, but within thirty seconds of irradiation, RNF8 protein tin be detected in clan with γH2AX.[71] RNF8 mediates extensive chromatin decondensation, through its subsequent interaction with CHD4,[72] a component of the nucleosome remodeling and deacetylase complex NuRD.
Pause for DNA repair [edit]
Later rapid chromatin remodeling, cell cycle checkpoints may be activated to allow DNA repair to be completed before the jail cell cycle progresses. First, two kinases, ATM and ATR, are activated within 5 or 6 minutes after Dna is damaged. This is followed by phosphorylation of the cell cycle checkpoint protein Chk1, initiating its office, about ten minutes after DNA is damaged.[73]
Role of oxidative damage to guanine in gene regulation [edit]
The Dna damage 8-oxo-dG does not occur randomly in the genome. In mouse embryonic fibroblasts, a 2 to 5-fold enrichment of 8-oxo-dG was found in genetic control regions, including promoters, 5'-untranslated regions and 3'-untranslated regions compared to 8-oxo-dG levels plant in cistron bodies and in intergenic regions.[74] In rat pulmonary artery endothelial cells, when 22,414 protein-coding genes were examined for locations of 8-oxo-dG, the bulk of 8-oxo-dGs (when present) were found in promoter regions rather than inside gene bodies.[75] Among hundreds of genes whose expression levels were affected by hypoxia, those with newly acquired promoter eight-oxo-dGs were upregulated, and those genes whose promoters lost 8-oxo-dGs were virtually all downregulated.[75]
Every bit reviewed by Wang et al.,[76] oxidized guanine appears to accept multiple regulatory roles in factor expression. In particular, when oxidative stress produces viii-oxo-dG in the promoter of a factor, the oxidative stress may also inactivate OGG1, an enzyme that targets 8-oxo-dG and normally initiates repair of eight-oxo-dG damage. The inactive OGG1, which no longer excises 8-oxo-dG, however targets and complexes with 8-oxo-dG, and causes a precipitous (~70o) bend in the Dna. This allows the associates of a transcriptional initiation complex, up-regulating transcription of the associated gene.[76] [77]
When viii-oxo-dG is formed in a guanine rich, potential G-quadruplex-forming sequence (PQS) in the coding strand of a promoter, active OGG1 excises the 8-oxo-dG and generates an apurinic/apyrimidinic site (AP site). The AP site enables melting of the duplex to unmask the PQS, adopting a G-quadruplex fold (G4 structure/motif) that has a regulatory office in transcription activation.[76] [78]
When eight-oxo-dG is complexed with active OGG1 it may then recruit chromatin remodelers to modulate cistron expression. Chromodomain helicase Dna-binding poly peptide 4 (CHD4), a component of the (NuRD) circuitous, is recruited by OGG1 to oxidative DNA damage sites. CHD4 then attracts Deoxyribonucleic acid and histone methylating enzymes that repress transcription of associated genes.[76]
Part of DNA damage in retention germination [edit]
Oxidation of guanine [edit]
Oxidation of guanine, particularly within CpG sites, may exist especially of import in learning and retention. Methylation of cytosines occurs at sixty–90% of CpG sites depending on the tissue type.[79] In the mammalian brain, ~62% of CpGs are methylated.[79] Methylation of CpG sites tends to stably silence genes.[eighty] More than 500 of these CpG sites are de-methylated in neuron Deoxyribonucleic acid during retentivity formation and memory consolidation in the hippocampus[81] [82] and cingulate cortex[82] regions of the brain. As indicated below, the first footstep in de-methylation of methylated cytosine at a CpG site is oxidation of the guanine to form 8-oxo-dG.
Function of oxidized guanine in Dna de-methylation [edit]
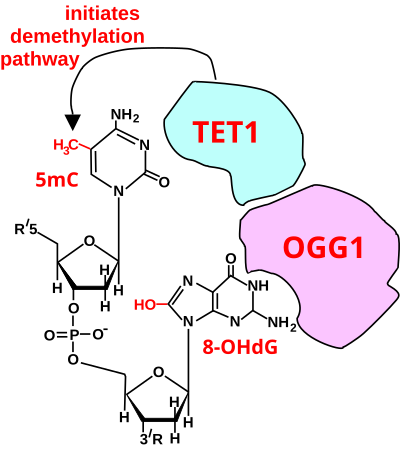
Initiation of Deoxyribonucleic acid demethylation at a CpG site. In adult somatic cells DNA methylation typically occurs in the context of CpG dinucleotides (CpG sites), forming 5-methylcytosine-pG, or 5mCpG. Reactive oxygen species (ROS) may attack guanine at the dinucleotide site, forming 8-hydroxy-two'-deoxyguanosine (8-OHdG), and resulting in a 5mCp-8-OHdG dinucleotide site. The base of operations excision repair enzyme OGG1 targets 8-OHdG and binds to the lesion without immediate excision. OGG1, nowadays at a 5mCp-eight-OHdG site recruits TET1 and TET1 oxidizes the 5mC adjacent to the viii-OHdG. This initiates demethylation of 5mC.[83]
The effigy in this department shows a CpG site where the cytosine is methylated to course 5-methylcytosine (5mC) and the guanine is oxidized to course 8-oxo-two'-deoxyguanosine (in the effigy this is shown in the tautomeric grade 8-OHdG). When this structure is formed, the base excision repair enzyme OGG1 targets 8-OHdG and binds to the lesion without immediate excision. OGG1, present at a 5mCp-8-OHdG site recruits TET1, and TET1 oxidizes the 5mC adjacent to the 8-OHdG. This initiates de-methylation of 5mC.[83] TET1 is a key enzyme involved in de-methylating 5mCpG. All the same, TET1 is only able to act on 5mCpG if the guanine was starting time oxidized to form 8-hydroxy-2'-deoxyguanosine (8-OHdG or its tautomer 8-oxo-dG), resulting in a 5mCp-viii-OHdG dinucleotide (see figure in this section).[83] This initiates the de-methylation pathway on the methylated cytosine, finally resulting in an unmethylated cytosine (see DNA oxidation for further steps in forming unmethylated cytosine).
Altered protein expression in neurons, due to changes in methylation of Deoxyribonucleic acid, (likely controlled past eight-oxo-dG-dependent de-methylation of CpG sites in gene promoters within neuron Deoxyribonucleic acid) has been established equally key to memory germination.[84]
Role of double-strand breaks in memory germination [edit]
Generation of Neuronal Activeness-Related DSBs
Double-stranded breaks (DSBs) in regions of Deoxyribonucleic acid related to neuronal activity are produced by a variety of mechanisms within and around the genome. The enzyme topoisomerase 2, or TOPIIβ plays a key function in DSB formation by aiding in the demethylation or loosening of histones wrapped around the double helix to promote transcription.[85] Once the chromatin construction is opened, DSBs are more than probable to accrue, yet, this is normally repaired by TOPIIβ through its intrinsic religation ability that rejoins the cleaved DNA ends.[85]
Failure of TOPIIβ to religase can accept drastic consequences on protein synthesis, where information technology is estimated that "blocking TOPIIβ activity alters the expression of nigh i-third of all developmentally regulated genes," such as neural immediate early genes (IEGs) involved in retentivity consolidation.[85] [86] Rapid expression of egr-i, c-Fos, and Arc IEGs accept been observed in response to increased neuronal action in the hippocampus region of the encephalon where memory processing takes place.[87] As a preventative measure out against TOPIIβ failure, DSB repair molecules are recruited via two dissimilar pathways: non-homologous end joining (NHEJ) pathway factors, which perform a similar religation part to that of TOPIIβ, and the homologous recombination (HR) pathway, which uses the non-broken sister strand as a template to repair the damaged strand of DNA.[85] [88]
Stimulation of neuronal activity, as previously mentioned in IEG expression, is some other mechanism through which DSBs are generated. Changes in level of activity accept been used in studies equally a biomarker to trace the overlap between DSBs and increased histone H3K4 methylation in promoter regions of IEGs.[85] [88] Other studies have indicated that transposable elements (TEs) can cause DSBs through endogenous action that involves using endonuclease enzymes to insert and cleave target Dna at random sites.[89] [90]
DSBs and Memory Reconsolidation
While accumulation of DSBs generally inhibits long term memory consolidation, the procedure of reconsolidation, in contrast, is DSB-dependent. Retentiveness reconsolidation involves the modification of existing memories stored in long-term memory.[91] Research involving NPAS4, a gene that regulates neuroplasticity in the hippocampus during contextual learning and memory formation, has revealed a link betwixt deletions in the coding region and impairments in recall of fear memories in transgenic rats.[85] Moreover, the enzyme H3K4me3, which catalyzes the demethylation of the H3K4 histone, was upregulated at the promoter region of the NPAS4 gene during the reconsolidation process, while knockdown (gene knockdown) of the same enzyme impeded reconsolidation.[85] A similar upshot was observed in TOPIIβ, where knockdown besides impaired the fear memory response in rats, indicating that DSBs, forth with the enzymes that regulate them, influence retention formation at multiple stages.
DSBs and Neurodegeneration
Buildup of DSBs more than broadly leads to the degeneration of neurons, hindering the function of memory and learning processes. Due to their lack of jail cell division and loftier metabolic activeness, neurons are especially prone to Deoxyribonucleic acid damage.[88] Additionally, an imbalance of DSBs and Deoxyribonucleic acid repair molecules for neuronal-activity genes has been linked to the evolution of various human neurodegenerative diseases including Alzheimer'south affliction (Advertisement), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS).[88] In patients with Alzheimer's disease, DSBs accumulate in neurons at early stages and are the driving force backside retentivity loss, a key characteristic of the disease.[88] Other external factors that effect in increased levels of activity-dependent DSBs in people with AD are oxidative damage to neurons, which can effect in more than DSBs when multiple lesions occur close to one another. Environmental factors such as viruses and a loftier-fat diet have besides been associated with disrupted function of Deoxyribonucleic acid repair molecules.
One targeted therapy for treating patients with Advertizing has involved suppression of the BRCA1 gene in man brains, initially tested in transgenic mice, where DSB levels were observed to have increased and memory loss had occurred, suggesting that BRCA1 could "serve as a therapeutic target for Advertisement and AD-related dementia." [88] Similarly, the poly peptide ATM involved in Dna repair and epigenetic modifications to the genome is positively correlated with neuronal loss in AD brains, indicating the protein is another key component in the intrinsically-linked processes of neurodegeneration, DSB product, and memory formation.[88]
Role of ATR and ATM [edit]
Nigh damage can exist repaired without triggering the impairment response system, notwithstanding more complex damage activates ATR and ATM, key protein kinases in the damage response system.[92] DNA damage inhibits M-CDKs which are a cardinal component of progression into Mitosis.
In all eukaryotic cells, ATR and ATM are protein kinases that detect DNA damage. They bind to DNA damaged sites and activate Chk1, Chk2, and, in fauna cells, p53. Together, these proteins brand up the DNA damage response organisation. Some DNA damage does not require the recruitment of ATR and ATM, it is just difficult and all-encompassing impairment that requires ATR and ATM. ATM and ATR are required for NHEJ, 60 minutes, ICL repair, and NER, as well as replication fork stability during unperturbed Deoxyribonucleic acid replication and in response to replication blocks.[7]
ATR is recruited for different forms of damage such as nucleotide damage, stalled replication forks and double strand breaks. ATM is specifically for the impairment response to double strand breaks. The MRN complex (equanimous of Mre11, Rad50, and Nbs1) course immediately at the site of double strand pause. This MRN circuitous recruits ATM to the site of damage. ATR and ATM phosphorylate various proteins that contribute to the impairment repair system. The binding of ATR and ATM to harm sites on DNA lead to the recruitment of Chk1 and Chk2. These protein kinases send damage signals to the cell cycle command organization to delay the progression of the prison cell bike.[thirteen]
Chk1 and Chk2 functions [edit]
Chk1 leads to the production of DNA repair enzymes. Chk2 leads to reversible cell bicycle abort. Chk2, besides as ATR/ATM, can actuate p53, which leads to permanent prison cell cycle abort or apoptosis.
p53 role in Dna impairment repair system [edit]
When there is too much damage, apoptosis is triggered in order to protect the organism from potentially harmful cells.vii p53, too known as a tumor suppressor gene, is a major regulatory protein in the DNA impairment response system which binds direct to the promoters of its target genes. p53 acts primarily at the G1 checkpoint (decision-making the G1 to South transition), where information technology blocks prison cell bike progression.[6] Activation of p53 tin can trigger jail cell death or permanent jail cell wheel abort. p53 can also activate certain repair pathways such was NER.[92]
Regulation of p53 [edit]
In the absence of Deoxyribonucleic acid damage, p53 is regulated by Mdm2 and constantly degraded. When there is DNA damage, Mdm2 is phosphorylated, most probable caused by ATM. The phosphorylation of Mdm2 leads to a reduction in the activeness of Mdm2, thus preventing the degradation of p53. Normal, undamaged cell, usually has low levels of p53 while cells under stress and DNA damage, will have high levels of p53.[13]
p53 serves every bit transcription cistron for bax and p21 [edit]
p53 serves as a transcription factors for both bax, a proapoptotic protein equally well as p21, a CDK inhibitor. CDK Inhibitors result in cell bicycle arrest. Arresting the cell provides the cell fourth dimension to repair the damage, and if the harm is irreparable, p53 recruits bax to trigger apoptosis.[92]
DDR and p53 role in cancer [edit]
p53 is a major central player in the growth of cancerous cells. Damaged Deoxyribonucleic acid cells with mutated p53 are at a college risk of becoming cancerous. Common chemotherapy treatments are genotoxic. These treatments are ineffective in cancer tumor that accept mutated p53 since they do not accept a performance p53 to either arrest or kill the damaged cell.
A major problem for life [edit]
One indication that Deoxyribonucleic acid damages are a major trouble for life is that Dna repair processes, to cope with DNA damages, have been found in all cellular organisms in which DNA repair has been investigated. For case, in bacteria, a regulatory network aimed at repairing DNA damages (called the SOS response in Escherichia coli) has been found in many bacterial species. E. coli RecA, a fundamental enzyme in the SOS response pathway, is the defining member of a ubiquitous course of DNA strand-exchange proteins that are essential for homologous recombination, a pathway that maintains genomic integrity by repairing cleaved DNA.[93] Genes homologous to RecA and to other cardinal genes in the SOS response pathway are plant in almost all the bacterial genomes sequenced to date, covering a large number of phyla, suggesting both an ancient origin and a widespread occurrence of recombinational repair of DNA damage.[94] Eukaryotic recombinases that are homologues of RecA are as well widespread in eukaryotic organisms. For case, in fission yeast and humans, RecA homologues promote duplex-duplex Dna-strand exchange needed for repair of many types of Dna lesions.[95] [96]
Another indication that DNA damages are a major problem for life is that cells make big investments in Dna repair processes. As pointed out by Hoeijmakers,[three] repairing just one double-strand interruption could require more than than ten,000 ATP molecules, as used in signaling the presence of the damage, the generation of repair foci, and the germination (in humans) of the RAD51 nucleofilament (an intermediate in homologous recombinational repair). (RAD51 is a homologue of bacterial RecA.) If the structural modification occurs during the G1 stage of DNA replication, the G1-S checkpoint arrests or postpones the furtherance of the cell cycle before the production enters the S phase.[1]
Consequences [edit]
Differentiated somatic cells of adult mammals more often than not replicate infrequently or non at all. Such cells, including, for example, brain neurons and muscle myocytes, have little or no jail cell turnover. Non-replicating cells do not generally generate mutations due to DNA damage-induced errors of replication. These non-replicating cells do non commonly give rise to cancer, merely they do accrue DNA damages with time that likely contribute to crumbling ( ). In a non-replicating prison cell, a unmarried-strand intermission or other type of damage in the transcribed strand of Dna can cake RNA polymerase Two-catalysed transcription.[97] This would interfere with the synthesis of the protein coded for past the gene in which the blockage occurred.
Brasnjevic et al.[98] summarized the evidence showing that unmarried-strand breaks accrue with historic period in the brain (though aggregating differed in dissimilar regions of the brain) and that single-strand breaks are the almost frequent steady-state DNA amercement in the brain. Equally discussed above, these accumulated single-strand breaks would be expected to block transcription of genes. Consistent with this, as reviewed past Hetman et al.,[99] 182 genes were identified and shown to have reduced transcription in the brains of individuals older than 72 years, compared to transcription in the brains of those less than 43 years old. When forty item proteins were evaluated in a muscle of rats, the majority of the proteins showed significant decreases during aging from 18 months (mature rat) to 30 months (aged rat) of age.[100]
Another type of DNA damage, the double-strand break, was shown to cause cell decease (loss of cells) through apoptosis.[101] This blazon of Deoxyribonucleic acid damage would not accumulate with age, since one time a prison cell was lost through apoptosis, its double-strand damage would exist lost with it. Thus, damaged DNA segments undermine the DNA replication machinery because these contradistinct sequences of DNA cannot be utilized as true templates to produce copies of ane'due south genetic material.[i]
RAD genes and the cell cycle response to DNA damage in Saccharomyces cerevisiae [edit]
When DNA is damaged, the jail cell responds in various ways to fix the damage and minimize the effects on the cell. One such response, specifically in eukaryotic cells, is to filibuster cell partition—the cell becomes arrested for some time in the G2 stage before progressing through the balance of the prison cell cycle. Diverse studies accept been conducted to elucidate the purpose of this G2 arrest that is induced by DNA damage. Researchers have found that cells that are prematurely forced out of the delay accept lower cell viability and higher rates of damaged chromosomes compared with cells that are able to undergo a total G2 arrest, suggesting that the purpose of the delay is to give the prison cell time to repair damaged chromosomes before continuing with the cell cycle.[102] This ensures the proper functioning of mitosis.
Various species of animals exhibit like mechanisms of cellular delay in response to Deoxyribonucleic acid harm, which tin can be caused by exposure to 10-irradiation. The budding yeast Saccharomyces cerevisiae has specifically been studied because progression through the prison cell bicycle can be followed via nuclear morphology with ease. By studying Saccharomyces cerevisiae, researchers have been able to learn more than about radiation-sensitive (RAD) genes, and the effect that RAD mutations may have on the typical cellular DNA damaged-induced delay response. Specifically, the RAD9 cistron plays a crucial role in detecting Deoxyribonucleic acid damage and arresting the cell in G2 until the damage is repaired.
Through all-encompassing experiments, researchers have been able to illuminate the role that the RAD genes play in delaying jail cell division in response to DNA damage. When wild-type, growing cells are exposed to diverse levels of x-irradiation over a given time frame, and so analyzed with a microcolony assay, differences in the jail cell cycle response can be observed based on which genes are mutated in the cells. For instance, while unirradiated cells will progress normally through the cell wheel, cells that are exposed to x-irradiation either permanently abort (go inviable) or delay in the G2 phase before standing to split in mitosis, further corroborating the thought that the G2 delay is crucial for DNA repair. However, rad strains, which are deficient in Dna repair, exhibit a markedly different response. For case, rad52 cells, which cannot repair double-stranded Dna breaks, tend to permanently arrest in G2 when exposed to even very low levels of x-irradiation, and rarely cease upwardly progressing through the afterward stages of the cell cycle. This is because the cells cannot repair Dna damage and thus do not enter mitosis. Various other rad mutants exhibit similar responses when exposed to ten-irradiation.
Yet, the rad9 strain exhibits an entirely different effect. These cells fail to filibuster in the G2 phase when exposed to 10-irradiation, and cease up progressing through the jail cell cycle unperturbed, before dying. This suggests that the RAD9 gene, unlike the other RAD genes, plays a crucial role in initiating G2 arrest. To further investigate these findings, the cell cycles of double mutant strains have been analyzed. A mutant rad52 rad9 strain—which is both defective in Deoxyribonucleic acid repair and G2 abort—fails to undergo prison cell bike arrest when exposed to x-irradiation. This suggests that even if DNA damage cannot be repaired, if RAD9 is not present, the cell cycle volition not delay. Thus, unrepaired DNA harm is the bespeak that tells RAD9 to halt division and arrest the cell bike in G2. Furthermore, at that place is a dose-dependent response; as the levels of 10-irradiation—and subsequent Dna damage—increase, more than cells, regardless of the mutations they take, become arrested in G2.
Some other, and maybe more helpful way to visualize this event is to await at photomicroscopy slides. Initially, slides of RAD+ and rad9 haploid cells in the exponential stage of growth testify simple, single cells, that are indistinguishable from each other. However, the slides look much different after being exposed to ten-irradiation for x hours. The RAD+ slides now prove RAD+ cells existing primarily as two-budded microcolonies, suggesting that cell sectionalisation has been arrested. In dissimilarity, the rad9 slides testify the rad9 cells existing primarily as 3 to viii budded colonies, and they appear smaller than the RAD+ cells. This is further show that the mutant RAD cells continued to dissever and are deficient in G2 abort.
Yet, there is evidence that although the RAD9 cistron is necessary to induce G2 arrest in response to DNA damage, giving the cell time to repair the damage, it does not really play a direct role in repairing Dna. When rad9 cells are artificially arrested in G2 with MBC, a microtubule poison that prevents cellular segmentation, and and so treated with 10-irradiation, the cells are able to repair their Dna and eventually progress through the cell cycle, dividing into viable cells. Thus, the RAD9 gene plays no function in really repairing damaged DNA—it simply senses damaged DNA and responds past delaying jail cell sectionalisation. The delay, then, is mediated by a control machinery, rather than the concrete damaged Deoxyribonucleic acid.[103]
On the other hand, information technology is possible that there are backup mechanisms that make full the role of RAD9 when it is not present. In fact, some studies have found that RAD9 does indeed play a critical role in DNA repair. In one written report, rad9 mutant and normal cells in the exponential phase of growth were exposed to UV-irradiation and synchronized in specific phases of the jail cell cycle. After being incubated to permit Dna repair, the extent of pyrimidine dimerization (which is indicative of Dna impairment) was assessed using sensitive primer extension techniques. It was found that the removal of Deoxyribonucleic acid photolesions was much less efficient in rad9 mutant cells than normal cells, providing evidence that RAD9 is involved in Dna repair. Thus, the role of RAD9 in repairing Dna impairment remains unclear.[104]
Regardless, it is clear that RAD9 is necessary to sense DNA damage and halt cell partition. RAD9 has been suggested to possess 3' to 5' exonuclease action, which is perchance why information technology plays a role in detecting DNA harm. When DNA is damaged, it is hypothesized that RAD9 forms a circuitous with RAD1 and HUS1, and this complex is recruited to sites of Deoxyribonucleic acid impairment. It is in this manner that RAD9 is able to exert its effects.
Although the office of RAD9 has primarily been studied in the budding yeast Saccharomyces cerevisiae, many of the cell cycle control mechanisms are like between species. Thus, we tin can conclude that RAD9 probable plays a critical role in the DNA harm response in humans too.
See also [edit]
- Ageing
- Crumbling brain
- AP site
- Direct Dna impairment
- Dna
- Dna adduct
- Deoxyribonucleic acid damage theory of crumbling
- Deoxyribonucleic acid repair
- DNA replication
- Free radical damage to DNA
- Homologous recombination
- Meiosis
- Mutation
- Natural competence
- Origin and function of meiosis
- Reactive oxygen species
References [edit]
- ^ a b c Köhler K, Ferreira P, Pfander B, Boos D (2016). The Initiation of DNA Replication in Eukaryotes. Springer, Cham. pp. 443–460. doi:ten.1007/978-3-319-24696-3_22. ISBN9783319246949.
- ^ Bernstein H, Payne CM, Bernstein C, Garewal H, Dvorak M (2008). Cancer and aging as consequences of united nations-repaired DNA damage. In: New Enquiry on Deoxyribonucleic acid Damages (Editors: Honoka Kimura and Aoi Suzuki) Nova Science Publishers, Inc., New York, Chapter i, pp. 1–47. open up access, but read but https://www.novapublishers.com/catalog/product_info.php?products_id=43247 Archived 2014-10-25 at the Wayback Machine ISBN 978-1604565812
- ^ a b Hoeijmakers JH (October 2009). "Deoxyribonucleic acid impairment, crumbling, and cancer". The New England Periodical of Medicine. 361 (xv): 1475–85. doi:ten.1056/NEJMra0804615. PMID 19812404.
- ^ Freitas AA, de Magalhães JP (2011). "A review and appraisal of the Dna damage theory of ageing". Mutation Research. 728 (1–2): 12–22. doi:x.1016/j.mrrev.2011.05.001. PMID 21600302.
- ^ O'Hagan HM, Mohammad HP, Baylin SB (August 2008). "Double strand breaks can initiate factor silencing and SIRT1-dependent onset of DNA methylation in an exogenous promoter CpG island". PLOS Genetics. 4 (8): e1000155. doi:10.1371/journal.pgen.1000155. PMC2491723. PMID 18704159.
- ^ a b "Khan Academy". Khan University . Retrieved 2017-12-15 .
- ^ a b Ciccia A, Elledge SJ (October 2010). "The Deoxyribonucleic acid damage response: making it rubber to play with knives". Molecular Jail cell. xl (ii): 179–204. doi:10.1016/j.molcel.2010.09.019. PMC2988877. PMID 20965415.
- ^ De Bont R, van Larebeke N (May 2004). "Endogenous Dna damage in humans: a review of quantitative information". Mutagenesis. 19 (3): 169–85. doi:10.1093/mutage/geh025. PMID 15123782.
- ^ a b c Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):7915-22. doi: 10.1073/pnas.90.17.7915. PMID 8367443; PMCID: PMC47258.
- ^ Yu Y, Cui Y, Niedernhofer LJ, Wang Y (December 2016). "Occurrence, Biological Consequences, and Human Wellness Relevance of Oxidative Stress-Induced Dna Damage". Chemical Research in Toxicology. 29 (12): 2008–2039. doi:10.1021/acs.chemrestox.6b00265. PMC5614522. PMID 27989142.
- ^ Dizdaroglu M, Coskun Due east, Jaruga P (May 2015). "Measurement of oxidatively induced Dna damage and its repair, by mass spectrometric techniques". Free Radical Inquiry. 49 (5): 525–48. doi:10.3109/10715762.2015.1014814. PMID 25812590. S2CID 31852987.
- ^ Lan L, Nakajima S, Oohata Y, Takao Chiliad, Okano S, Masutani Yard, et al. (September 2004). "In situ analysis of repair processes for oxidative Dna damage in mammalian cells". Proceedings of the National Academy of Sciences of the United States of America. 101 (38): 13738–43. Bibcode:2004PNAS..10113738L. doi:x.1073/pnas.0406048101. PMC518826. PMID 15365186.
- ^ a b c Morgan, David (2006). Jail cell Cycle: Principles of Command. London: New Science Press.
- ^ a b c Helbock HJ, Beckman KB, Shigenaga MK, Walter PB, Woodall AA, Yeo HC, Ames BN (January 1998). "Dna oxidation matters: the HPLC-electrochemical detection assay of eight-oxo-deoxyguanosine and 8-oxo-guanine". Proceedings of the National Academy of Sciences of the United States of America. 95 (one): 288–93. Bibcode:1998PNAS...95..288H. doi:10.1073/pnas.95.1.288. PMC18204. PMID 9419368.
- ^ a b Foksinski M, Rozalski R, Guz J, Ruszkowska B, Sztukowska P, Piwowarski Grand, et al. (November 2004). "Urinary excretion of DNA repair products correlates with metabolic rates every bit well every bit with maximum life spans of dissimilar mammalian species". Gratuitous Radical Biological science & Medicine. 37 (9): 1449–54. doi:10.1016/j.freeradbiomed.2004.07.014. PMID 15454284.
- ^ a b Tudek B, Winczura A, Janik J, Siomek A, Foksinski M, Oliński R (May 2010). "Involvement of oxidatively damaged DNA and repair in cancer evolution and aging". American Periodical of Translational Research. 2 (iii): 254–84. PMC2892402. PMID 20589166.
- ^ Fraga CG, Shigenaga MK, Park JW, Degan P, Ames BN (June 1990). "Oxidative harm to DNA during crumbling: 8-hydroxy-2'-deoxyguanosine in rat organ Dna and urine". Proceedings of the National Academy of Sciences of the United States of America. 87 (12): 4533–7. Bibcode:1990PNAS...87.4533F. doi:ten.1073/pnas.87.12.4533. PMC54150. PMID 2352934.
- ^ a b c Hamilton ML, Guo Z, Fuller CD, Van Remmen H, Ward WF, Austad SN, et al. (May 2001). "A reliable cess of 8-oxo-2-deoxyguanosine levels in nuclear and mitochondrial DNA using the sodium iodide method to isolate DNA". Nucleic Acids Research. 29 (ten): 2117–26. doi:x.1093/nar/29.ten.2117. PMC55450. PMID 11353081.
- ^ Lindahl T, Nyberg B (September 1972). "Rate of depurination of native deoxyribonucleic acrid". Biochemistry. 11 (19): 3610–eight. doi:10.1021/bi00769a018. PMID 4626532.
- ^ Lindahl T (April 1993). "Instability and decay of the master structure of DNA". Nature. 362 (6422): 709–fifteen. Bibcode:1993Natur.362..709L. doi:10.1038/362709a0. PMID 8469282. S2CID 4283694.
- ^ Nakamura J, Walker VE, Upton PB, Chiang SY, Kow YW, Swenberg JA (January 1998). "Highly sensitive apurinic/apyrimidinic site analysis can discover spontaneous and chemically induced depurination nether physiological conditions". Cancer Research. 58 (2): 222–5. PMID 9443396.
- ^ a b Lindahl T. (1977) Deoxyribonucleic acid repair enzymes interim on spontaneous lesions in DNA. In: Nichols WW and White potato DG (eds.) DNA Repair Processes. Symposia Specialists, Miami p225-240. ISBN 088372099X ISBN 978-0883720998
- ^ a b c d due east Tice, R.R., and Setlow, R.B. (1985) Deoxyribonucleic acid repair and replication in aging organisms and cells. In: Finch EE and Schneider EL (eds.) Handbook of the Biology of Aging. Van Nostrand Reinhold, New York. Pages 173–224. ISBN 0442225296 ISBN 978-0442225292
- ^ Haber JE (July 1999). "Deoxyribonucleic acid recombination: the replication connection". Trends in Biochemical Sciences. 24 (seven): 271–5. doi:10.1016/s0968-0004(99)01413-9. PMID 10390616.
- ^ Vilenchik MM, Knudson AG (Oct 2003). "Endogenous DNA double-strand breaks: product, fidelity of repair, and induction of cancer". Proceedings of the National Academy of Sciences of the The states of America. 100 (22): 12871–6. Bibcode:2003PNAS..10012871V. doi:ten.1073/pnas.2135498100. PMC240711. PMID 14566050.
- ^ Chan SW, Dedon PC (December 2010). "The biological and metabolic fates of endogenous DNA damage products". Journal of Nucleic Acids. 2010: 929047. doi:10.4061/2010/929047. PMC3010698. PMID 21209721.
- ^ Kadlubar FF, Anderson KE, Häussermann S, Lang NP, Barone GW, Thompson PA, et al. (September 1998). "Comparison of Dna adduct levels associated with oxidative stress in homo pancreas". Mutation Inquiry. 405 (ii): 125–33. doi:10.1016/s0027-5107(98)00129-8. PMID 9748537.
- ^ VanderVeen LA, Hashim MF, Shyr Y, Marnett LJ (Nov 2003). "Induction of frameshift and base pair substitution mutations by the major DNA adduct of the endogenous carcinogen malondialdehyde". Proceedings of the National Academy of Sciences of the United States of America. 100 (24): 14247–52. Bibcode:2003PNAS..10014247V. doi:10.1073/pnas.2332176100. PMC283577. PMID 14603032.
- ^ Tan X, Grollman AP, Shibutani S (December 1999). "Comparison of the mutagenic properties of 8-oxo-seven,viii-dihydro-2'-deoxyadenosine and 8-oxo-vii,8-dihydro-two'-deoxyguanosine Dna lesions in mammalian cells". Carcinogenesis. 20 (12): 2287–92. doi:x.1093/carcin/xx.12.2287. PMID 10590221.
- ^ Swenberg JA, Lu G, Moeller BC, Gao L, Upton Lead, Nakamura J, Starr TB (March 2011). "Endogenous versus exogenous DNA adducts: their role in carcinogenesis, epidemiology, and chance assessment". Toxicological Sciences. 120 Suppl ane (Suppl 1): S130-45. doi:10.1093/toxsci/kfq371. PMC3043087. PMID 21163908.
- ^ Nakamura J, Swenberg JA (June 1999). "Endogenous apurinic/apyrimidinic sites in genomic DNA of mammalian tissues". Cancer Research. 59 (eleven): 2522–6. PMID 10363965.
- ^ a b c Xia J, Chiu LY, Nehring RB, Bravo Núñez MA, Mei Q, Perez Thou, et al. (January 2019). "Bacteria-to-Human Poly peptide Networks Reveal Origins of Endogenous Dna Damage". Cell. 176 (ane–2): 127–143.e24. doi:10.1016/j.cell.2018.12.008. PMC6344048. PMID 30633903.
- ^ Krokan HE, Bjørås M (Apr 2013). "Base of operations excision repair". Common cold Spring Harbor Perspectives in Biology. v (4): a012583. doi:ten.1101/cshperspect.a012583. PMC3683898. PMID 23545420.
- ^ del Rivero J, Kohn EC (April 2017). "PARP Inhibitors: The Cornerstone of Deoxyribonucleic acid Repair-Targeted Therapies". Oncology. 31 (4): 265–73. PMID 28412778.
- ^ Schärer OD (October 2013). "Nucleotide excision repair in eukaryotes". Cold Bound Harbor Perspectives in Biology. 5 (ten): a012609. doi:ten.1101/cshperspect.a012609. PMC3783044. PMID 24086042.
- ^ de Boer J, Hoeijmakers JH (March 2000). "Nucleotide excision repair and human syndromes". Carcinogenesis. 21 (iii): 453–lx. doi:10.1093/carcin/21.iii.453. PMID 10688865.
- ^ Satoh MS, Jones CJ, Wood RD, Lindahl T (July 1993). "DNA excision-repair defect of xeroderma pigmentosum prevents removal of a class of oxygen gratis radical-induced base lesions". Proceedings of the National Academy of Sciences of the United States of America. 90 (xiii): 6335–ix. Bibcode:1993PNAS...90.6335S. doi:ten.1073/pnas.90.xiii.6335. PMC46923. PMID 8327515.
- ^ a b c Ceccaldi R, Rondinelli B, D'Andrea AD (January 2016). "Repair Pathway Choices and Consequences at the Double-Strand Break". Trends in Cell Biology. 26 (ane): 52–64. doi:10.1016/j.tcb.2015.07.009. PMC4862604. PMID 26437586.
- ^ Kunkel TA, Erie DA (2005). "Deoxyribonucleic acid mismatch repair". Annual Review of Biochemistry. 74: 681–710. doi:ten.1146/annurev.biochem.74.082803.133243. PMID 15952900.
- ^ Yi C, He C (January 2013). "DNA repair by reversal of Dna damage". Cold Spring Harbor Perspectives in Biology. 5 (1): a012575. doi:10.1101/cshperspect.a012575. PMC3579392. PMID 23284047.
- ^ Lehmann AR (February 2005). "Replication of damaged Dna by translesion synthesis in homo cells". FEBS Letters. 579 (4): 873–6. doi:10.1016/j.febslet.2004.11.029. PMID 15680966. S2CID 38747288.
- ^ Nowsheen Due south, Yang ES (Oct 2012). "The intersection between Dna damage response and cell decease pathways". Experimental Oncology. 34 (3): 243–54. PMC3754840. PMID 23070009.
- ^ a b Bernstein C, Bernstein H, Payne CM, Garewal H (June 2002). "DNA repair/pro-apoptotic dual-role proteins in five major DNA repair pathways: fail-safe protection confronting carcinogenesis". Mutation Research. 511 (two): 145–78. doi:x.1016/s1383-5742(02)00009-1. PMID 12052432.
- ^ Deng T, Lyon CJ, Bergin Due south, Caligiuri MA, Hsueh WA (May 2016). "Obesity, Inflammation, and Cancer". Annual Review of Pathology. 11: 421–49. doi:10.1146/annurev-pathol-012615-044359. PMID 27193454.
- ^ a b Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA (Dec 2016). "Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation". Journal of Clinical Oncology. 34 (35): 4270–4276. doi:10.1200/JCO.2016.67.4283. PMC5562428. PMID 27903155.
- ^ Ramos-Nino ME (2013). "The role of chronic inflammation in obesity-associated cancers". ISRN Oncology. 2013: 697521. doi:10.1155/2013/697521. PMC3683483. PMID 23819063.
- ^ "Obesity and Cancer". 2017.
- ^ Coussens LM, Werb Z (2002). "Inflammation and cancer". Nature. 420 (6917): 860–seven. Bibcode:2002Natur.420..860C. doi:10.1038/nature01322. PMC2803035. PMID 12490959.
- ^ Chiba T, Marusawa H, Ushijima T (September 2012). "Inflammation-associated cancer development in digestive organs: mechanisms and roles for genetic and epigenetic modulation". Gastroenterology. 143 (iii): 550–563. doi:ten.1053/j.gastro.2012.07.009. hdl:2433/160134. PMID 22796521.
- ^ Shacter E, Weitzman SA (February 2002). "Chronic inflammation and cancer". Oncology. 16 (2): 217–26, 229, give-and-take 230–2. PMID 11866137.
- ^ Valgimigli M, Valgimigli L, Trerè D, Gaiani S, Pedulli GF, Gramantieri L, Bolondi L (September 2002). "Oxidative stress EPR measurement in human liver by radical-probe technique. Correlation with etiology, histology and prison cell proliferation". Free Radical Enquiry. 36 (ix): 939–48. doi:10.1080/107156021000006653. PMID 12448819. S2CID 12061790.
- ^ Hardbower DM, de Sablet T, Chaturvedi R, Wilson KT (2013). "Chronic inflammation and oxidative stress: the smoking gun for Helicobacter pylori-induced gastric cancer?". Gut Microbes. four (6): 475–81. doi:ten.4161/gmic.25583. PMC3928159. PMID 23811829.
- ^ Ernst P (March 1999). "Review commodity: the office of inflammation in the pathogenesis of gastric cancer". Comestible Pharmacology & Therapeutics. 13 Suppl 1: xiii–8. doi:x.1046/j.1365-2036.1999.00003.x. PMID 10209682. S2CID 38496014.
- ^ Marciani L, Cox EF, Hoad CL, Totman JJ, Costigan C, Singh G, et al. (November 2013). "Effects of various food ingredients on gall float emptying". European Journal of Clinical Nutrition. 67 (11): 1182–7. doi:x.1038/ejcn.2013.168. PMC3898429. PMID 24045793.
- ^ Payne CM, Bernstein C, Dvorak K, Bernstein H (2008). "Hydrophobic bile acids, genomic instability, Darwinian selection, and colon carcinogenesis". Clinical and Experimental Gastroenterology. i: 19–47. doi:10.2147/ceg.s4343. PMC3108627. PMID 21677822.
- ^ Bernstein C, Bernstein H, Garewal H, Dinning P, Jabi R, Sampliner RE, et al. (May 1999). "A bile acrid-induced apoptosis assay for colon cancer gamble and associated quality command studies". Cancer Research. 59 (ten): 2353–7. PMID 10344743.
- ^ Bernstein C, Holubec H, Bhattacharyya AK, Nguyen H, Payne CM, Zaitlin B, Bernstein H (August 2011). "Carcinogenicity of deoxycholate, a secondary bile acid". Archives of Toxicology. 85 (eight): 863–71. doi:10.1007/s00204-011-0648-7. PMC3149672. PMID 21267546.
- ^ Zhang Fifty, Yu J (December 2013). "Role of apoptosis in colon cancer biology, therapy, and prevention". Current Colorectal Cancer Reports. 9 (4): 331–340. doi:10.1007/s11888-013-0188-z. PMC3836193. PMID 24273467.
- ^ Williams GT, Critchlow MR, Hedge VL, O'Hare KB (December 1998). "Molecular failure of apoptosis: inappropriate cell survival and mutagenesis?". Toxicology Messages. 102–103: 485–9. doi:ten.1016/s0378-4274(98)00343-ix. PMID 10022300.
- ^ Liu B, Yip RK, Zhou Z (November 2012). "Chromatin remodeling, DNA impairment repair and crumbling". Current Genomics. xiii (7): 533–47. doi:10.2174/138920212803251373. PMC3468886. PMID 23633913.
- ^ "Human DNA repair genes".
- ^ a b Abdou I, Poirier GG, Hendzel MJ, Weinfeld M (Jan 2015). "DNA ligase III acts as a DNA strand break sensor in the cellular orchestration of DNA strand break repair". Nucleic Acids Enquiry. 43 (2): 875–92. doi:x.1093/nar/gku1307. PMC4333375. PMID 25539916.
- ^ a b Sellou H, Lebeaupin T, Chapuis C, Smith R, Hegele A, Singh 60 minutes, et al. (December 2016). "The poly(ADP-ribose)-dependent chromatin remodeler Alc1 induces local chromatin relaxation upon DNA damage". Molecular Biological science of the Prison cell. 27 (24): 3791–3799. doi:ten.1091/mbc.E16-05-0269. PMC5170603. PMID 27733626.
- ^ Luijsterburg MS, Goedhart J, Moser J, Kool H, Geverts B, Houtsmuller AB, et al. (August 2007). "Dynamic in vivo interaction of DDB2 E3 ubiquitin ligase with UV-damaged Dna is contained of damage-recognition protein XPC". Journal of Prison cell Science. 120 (Pt 15): 2706–16. doi:x.1242/jcs.008367. PMID 17635991.
- ^ a b Pines A, Vrouwe MG, Marteijn JA, Typas D, Luijsterburg MS, Cansoy M, et al. (October 2012). "PARP1 promotes nucleotide excision repair through DDB2 stabilization and recruitment of ALC1". The Journal of Cell Biology. 199 (ii): 235–49. doi:10.1083/jcb.201112132. PMC3471223. PMID 23045548.
- ^ Yeh JI, Levine As, Du S, Chinte U, Ghodke H, Wang H, et al. (October 2012). "Damaged Dna induced UV-damaged Deoxyribonucleic acid-binding protein (UV-DDB) dimerization and its roles in chromatinized DNA repair". Proceedings of the National University of Sciences of the U.s.a.. 109 (41): E2737-46. doi:10.1073/pnas.1110067109. PMC3478663. PMID 22822215.
- ^ Jiang Y, Wang X, Bao Southward, Guo R, Johnson DG, Shen X, Li L (October 2010). "INO80 chromatin remodeling circuitous promotes the removal of UV lesions by the nucleotide excision repair pathway". Proceedings of the National University of Sciences of the United States of America. 107 (40): 17274–9. Bibcode:2010PNAS..10717274J. doi:10.1073/pnas.1008388107. PMC2951448. PMID 20855601.
- ^ a b c Van Meter Chiliad, Simon M, Tombline K, May A, Morello TD, Hubbard BP, et al. (September 2016). "JNK Phosphorylates SIRT6 to Stimulate DNA Double-Strand Break Repair in Response to Oxidative Stress past Recruiting PARP1 to DNA Breaks". Prison cell Reports. 16 (10): 2641–2650. doi:x.1016/j.celrep.2016.08.006. PMC5089070. PMID 27568560.
- ^ a b Haince JF, McDonald D, Rodrigue A, Déry U, Masson JY, Hendzel MJ, Poirier GG (January 2008). "PARP1-dependent kinetics of recruitment of MRE11 and NBS1 proteins to multiple Deoxyribonucleic acid damage sites". The Journal of Biological Chemistry. 283 (2): 1197–208. doi:ten.1074/jbc.M706734200. PMID 18025084.
- ^ a b c Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM (March 1998). "Deoxyribonucleic acid double-stranded breaks induce histone H2AX phosphorylation on serine 139". The Journal of Biological Chemical science. 273 (10): 5858–68. doi:10.1074/jbc.273.10.5858. PMID 9488723.
- ^ Mailand N, Bekker-Jensen Due south, Faustrup H, Melander F, Bartek J, Lukas C, Lukas J (November 2007). "RNF8 ubiquitylates histones at Deoxyribonucleic acid double-strand breaks and promotes assembly of repair proteins". Prison cell. 131 (5): 887–900. doi:10.1016/j.cell.2007.09.040. PMID 18001824. S2CID 14232192.
- ^ Luijsterburg MS, Acs K, Ackermann Fifty, Wiegant WW, Bekker-Jensen South, Larsen DH, et al. (May 2012). "A new non-catalytic role for ubiquitin ligase RNF8 in unfolding higher-order chromatin structure". The EMBO Periodical. 31 (11): 2511–27. doi:ten.1038/emboj.2012.104. PMC3365417. PMID 22531782.
- ^ Jazayeri A, Falck J, Lukas C, Bartek J, Smith GC, Lukas J, Jackson SP (January 2006). "ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks". Nature Cell Biological science. 8 (1): 37–45. doi:10.1038/ncb1337. PMID 16327781. S2CID 9797133.
- ^ Ding Y, Fleming AM, Burrows CJ (February 2017). "Sequencing the Mouse Genome for the Oxidatively Modified Base eight-Oxo-7,8-dihydroguanine past OG-Seq". Journal of the American Chemic Society. 139 (vii): 2569–2572. doi:ten.1021/jacs.6b12604. PMC5440228. PMID 28150947.
- ^ a b Pastukh Five, Roberts JT, Clark DW, Bardwell GC, Patel M, Al-Mehdi AB, et al. (Dec 2015). "An oxidative DNA "damage" and repair mechanism localized in the VEGF promoter is important for hypoxia-induced VEGF mRNA expression". American Periodical of Physiology. Lung Cellular and Molecular Physiology. 309 (11): L1367-75. doi:ten.1152/ajplung.00236.2015. PMC4669343. PMID 26432868.
- ^ a b c d Wang R, Hao W, Pan Fifty, Boldogh I, Ba Ten (October 2018). "The roles of base excision repair enzyme OGG1 in gene expression". Cellular and Molecular Life Sciences. 75 (xx): 3741–3750. doi:x.1007/s00018-018-2887-viii. PMC6154017. PMID 30043138.
- ^ Seifermann M, Epe B (June 2017). "Oxidatively generated base of operations modifications in DNA: Non just carcinogenic risk factor merely also regulatory mark?". Free Radical Biology & Medicine. 107: 258–265. doi:ten.1016/j.freeradbiomed.2016.11.018. PMID 27871818.
- ^ Fleming AM, Burrows CJ (Baronial 2017). "viii-Oxo-7,eight-dihydroguanine, friend and foe: Epigenetic-similar regulator versus initiator of mutagenesis". DNA Repair. 56: 75–83. doi:x.1016/j.dnarep.2017.06.009. PMC5548303. PMID 28629775.
- ^ a b Fasolino Chiliad, Zhou Z (May 2017). "The Crucial Office of DNA Methylation and MeCP2 in Neuronal Office". Genes. 8 (5): 141. doi:10.3390/genes8050141. PMC5448015. PMID 28505093.
- ^ Bird A (Jan 2002). "Dna methylation patterns and epigenetic memory". Genes & Development. 16 (one): vi–21. doi:10.1101/gad.947102. PMID 11782440.
- ^ Duke CG, Kennedy AJ, Gavin CF, Day JJ, Sweatt JD (July 2017). "Experience-dependent epigenomic reorganization in the hippocampus". Learning & Memory. 24 (seven): 278–288. doi:10.1101/lm.045112.117. PMC5473107. PMID 28620075.
- ^ a b Halder R, Hennion 1000, Vidal RO, Shomroni O, Rahman RU, Rajput A, et al. (Jan 2016). "DNA methylation changes in plasticity genes back-trail the formation and maintenance of memory". Nature Neuroscience. 19 (i): 102–x. doi:ten.1038/nn.4194. PMC4700510. PMID 26656643.
- ^ a b c Zhou X, Zhuang Z, Wang W, He Fifty, Wu H, Cao Y, et al. (September 2016). "OGG1 is essential in oxidative stress induced Deoxyribonucleic acid demethylation". Cellular Signalling. 28 (9): 1163–71. doi:10.1016/j.cellsig.2016.05.021. PMID 27251462.
- ^ Twenty-four hours JJ, Sweatt JD (November 2010). "Deoxyribonucleic acid methylation and memory formation". Nature Neuroscience. 13 (xi): 1319–23. doi:10.1038/nn.2666. PMC3130618. PMID 20975755.
- ^ a b c d e f g Navabpour, Shaghayegh; Rogers, Jessie; McFadden, Taylor; Jarome, Timothy J. (2020-xi-26). "DNA Double-Strand Breaks Are a Critical Regulator of Fear Retentiveness Reconsolidation". International Periodical of Molecular Sciences. 21 (23): 8995. doi:10.3390/ijms21238995. ISSN 1422-0067. PMC7730899. PMID 33256213.
- ^ Li, Xiang; Marshall, Paul R.; Leighton, Laura J.; Zajaczkowski, Esmi 50.; Wang, Ziqi; Madugalle, Sachithrani U.; Yin, Jiayu; Bredy, Timothy W.; Wei, Wei (2019-02-06). "The Deoxyribonucleic acid Repair-Associated Protein Gadd45γ Regulates the Temporal Coding of Firsthand Early Gene Expression within the Prelimbic Prefrontal Cortex and Is Required for the Consolidation of Associative Fear Memory". The Journal of Neuroscience. 39 (6): 970–983. doi:10.1523/JNEUROSCI.2024-eighteen.2018. ISSN 0270-6474. PMC6363930. PMID 30545945.
- ^ Minatohara, Keiichiro; Akiyoshi, Mika; Okuno, Hiroyuki (2016-01-05). "Role of Immediate-Early Genes in Synaptic Plasticity and Neuronal Ensembles Underlying the Retention Trace". Frontiers in Molecular Neuroscience. 8: 78. doi:ten.3389/fnmol.2015.00078. ISSN 1662-5099. PMC4700275. PMID 26778955.
- ^ a b c d e f g Thadathil, Nidheesh; Hori, Roderick; Xiao, Jianfeng; Khan, Mohammad Moshahid (December 2019). "Deoxyribonucleic acid double-strand breaks: a potential therapeutic target for neurodegenerative diseases". Chromosome Research. 27 (4): 345–364. doi:10.1007/s10577-019-09617-x. ISSN 0967-3849. PMC7934912. PMID 31707536.
- ^ Gasior, Stephen Fifty.; Wakeman, Timothy P.; Xu, Bo; Deininger, Prescott L. (April 2006). "The Human LINE-ane Retrotransposon Creates Dna Double-strand Breaks". Journal of Molecular Biological science. 357 (5): 1383–1393. doi:10.1016/j.jmb.2006.01.089. PMC4136747. PMID 16490214.
- ^ Shanbhag, Niraj M.; Evans, Mark D.; Mao, Wenjie; Nana, Alissa L.; Seeley, William W.; Adame, Anthony; Rissman, Robert A.; Masliah, Eliezer; Mucke, Lennart (December 2019). "Early neuronal aggregating of Deoxyribonucleic acid double strand breaks in Alzheimer's affliction". Acta Neuropathologica Communications. 7 (1): 77. doi:10.1186/s40478-019-0723-5. ISSN 2051-5960. PMC6524256. PMID 31101070.
- ^ Tronson, Natalie C.; Taylor, Jane R. (April 2007). "Molecular mechanisms of retentivity reconsolidation". Nature Reviews Neuroscience. eight (4): 262–275. doi:10.1038/nrn2090. ISSN 1471-003X. PMID 17342174. S2CID 1835412.
- ^ a b c Giglia-Mari G, Zotter A, Vermeulen W (January 2011). "Deoxyribonucleic acid damage response". Cold Leap Harbor Perspectives in Biology. 3 (1): a000745. doi:10.1101/cshperspect.a000745. PMC3003462. PMID 20980439.
- ^ Bell JC, Plank JL, Dombrowski CC, Kowalczykowski SC (November 2012). "Direct imaging of RecA nucleation and growth on unmarried molecules of SSB-coated ssDNA". Nature. 491 (7423): 274–8. Bibcode:2012Natur.491..274B. doi:x.1038/nature11598. PMC4112059. PMID 23103864.
- ^ Erill I, Campoy S, Barbé J (2007). "Aeons of distress: an evolutionary perspective on the bacterial SOS response". FEMS Microbiol. Rev. 31 (6): 637–656. doi:x.1111/j.1574-6976.2007.00082.x. PMID 17883408.
- ^ Murayama Y, Kurokawa Y, Mayanagi K, Iwasaki H (Feb 2008). "Formation and branch migration of Holliday junctions mediated by eukaryotic recombinases". Nature. 451 (7181): 1018–21. Bibcode:2008Natur.451.1018M. doi:10.1038/nature06609. PMID 18256600. S2CID 205212254.
- ^ Holthausen JT, Wyman C, Kanaar R (2010). "Regulation of DNA strand exchange in homologous recombination". DNA Repair (Amst). ix (12): 1264–1272. doi:x.1016/j.dnarep.2010.09.014. PMID 20971042.
- ^ Kathe SD, Shen GP, Wallace SS (April 2004). "Single-stranded breaks in DNA simply not oxidative DNA base of operations damages cake transcriptional elongation past RNA polymerase 2 in HeLa cell nuclear extracts". The Periodical of Biological Chemical science. 279 (18): 18511–20. doi:10.1074/jbc.M313598200. PMID 14978042.
- ^ Brasnjevic I, Hof PR, Steinbusch HW, Schmitz C (2008). "Accumulation of nuclear DNA damage or neuron loss: molecular ground for a new approach to understanding selective neuronal vulnerability in neurodegenerative diseases". DNA Repair (Amst.). 7 (vii): 1087–1097. doi:ten.1016/j.dnarep.2008.03.010. PMC2919205. PMID 18458001.
- ^ Hetman M, Vashishta A, Rempala 1000 (2010). "Neurotoxic mechanisms of DNA damage: focus on transcriptional inhibition". J. Neurochem. 114 (half dozen): 1537–1549. doi:10.1111/j.1471-4159.2010.06859.x. PMC2945429. PMID 20557419.
- ^ Piec I, Listrat A, Alliot J, Chambon C, Taylor RG, Bechet D (July 2005). "Differential proteome analysis of aging in rat skeletal muscle". FASEB Periodical. xix (ix): 1143–five. doi:ten.1096/fj.04-3084fje. PMID 15831715. S2CID 33187815.
- ^ Carnevale J, Palander O, Seifried LA, Dick FA (March 2012). "Deoxyribonucleic acid damage signals through differentially modified E2F1 molecules to induce apoptosis". Molecular and Cellular Biology. 32 (5): 900–12. doi:10.1128/MCB.06286-11. PMC3295199. PMID 22184068.
- ^ Hittelman WN, Rao PN (1975). "Mutat. Res. 23 1974; 251; A.P. Rao and P.N. Rao, J. Natl. Cancer Inst. 57 1976; 1139; W.Northward. Hittelman and P.N. Rao, Cancer Res. 34 1974; 3433;". 35: 3027.
- ^ Weinert TA, Hartwell LH (July 1988). "The RAD9 gene controls the cell cycle response to Deoxyribonucleic acid damage in Saccharomyces cerevisiae". Scientific discipline. 241 (4863): 317–22. Bibcode:1988Sci...241..317W. doi:ten.1126/science.3291120. PMID 3291120. S2CID 36645009.
- ^ Al-Moghrabi NM, Al-Sharif IS, Aboussekhra A (May 2001). "The Saccharomyces cerevisiae RAD9 cell cycle checkpoint gene is required for optimal repair of UV-induced pyrimidine dimers in both Chiliad(1) and G(2)/G phases of the jail cell bicycle". Nucleic Acids Research. 29 (10): 2020–5. doi:10.1093/nar/29.10.2020. PMC55462. PMID 11353070.
Source: https://en.wikipedia.org/wiki/DNA_damage_%28naturally_occurring%29
Posted by: sargentthoreeduck.blogspot.com


0 Response to "What Are Damages Or Changes To A Dna Sequence Called"
Post a Comment